22+ How To Calculate Osmoles
A 1M solution of NaCl 2 Osm. To calculate osmolarity in a solution it is necessary to calculate the number of osmoles in a.

Hyperosmolar Hyperglycemic State Hhs Emcrit Project
465 62 votes Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity.
. 1 x 3 3 osmol. You multiply the molarity by the number of osmoles that each solute produces. For instance if your have a 1 mol solution of MgCl2.
A solution of 1 molL NaCl has an osmolarity of 2 OsmolL. For instance if your have. If we dissolve MgCl 2 in water it dissociates into 3 ions Mg and 2 Cl one ion of Magnesium and two ions of Chloride.
For example a 1M solution of a nonionizing substance such as glucose is a 1 Osmolar solution. Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity osmol. How do you calculate mOsm from mEq.
How do you calculate the osmolarity of NaCl. An osmole Osmol is 1 mol of particles that contribute to the osmotic pressure of a solution. 465 62 votes.
One mole of Na and one mole of Cl. How to calculate osmoles. You multiply the molarity by the.
For example a 1M solution of a nonionizing substance such as glucose is a 1 Osmolar solution. How do you calculate the osmolarity of NaCl. Lastly our body fluids molarity is about 015 M and.
Thus each mole of NaCl becomes two osmoles in solution. A 1M solution of NaCl 2 Osm. One mole of Na and one mole of.
How do you calculate the osmolarity of NaCl. READ THIS BEFORE WATCHING THE VIDEO. For example NaCl dissociates completely in water to form Na ions and Cl ions.
According to Wikpedia Plasma osmolarity measures the bodys electrolyte-water balance An increase in plasma osmolarity can be a sign of dehydration or disease. The osmolarity of intravenous saline solutions can be easily calculated by multiplying the sodium and potassium. The molecular mass of Cl 354 g.
Urine osmolarity allows the etiological search for a hydroelectrolyte disorder. Thus each mole of NaCl becomes two osmoles in solution. Urine osmolarity is very variable and depends on the pathology of the patient.
Calculate the osmolarity of 0140. A 1M solution of NaCl 2. The question in Example 2 should read.
The molecular mass of Na 229 g. Osmolarity represents the number of miliosoles per liter of solution. This video illustrates how to solve osmolarity calculation problems.
How do you calculate the osmolarity of a solution. For example a 1M solution of a nonionizing substance such as glucose is a 1 Osmolar solution. Multiply the number of particles produced from dissolving the solution in water by the molarity to find the osmolarity osmol.
How do you calculate osmoles. MDCalc loves calculator creators researchers who through intelligent and often complex methods discover tools that describe scientific facts that can then be applied in practice. Osmolarity is similar but is defined as the number of osmoles or mOsmol per liter of solvent.

Bldt2qqe34ct7m
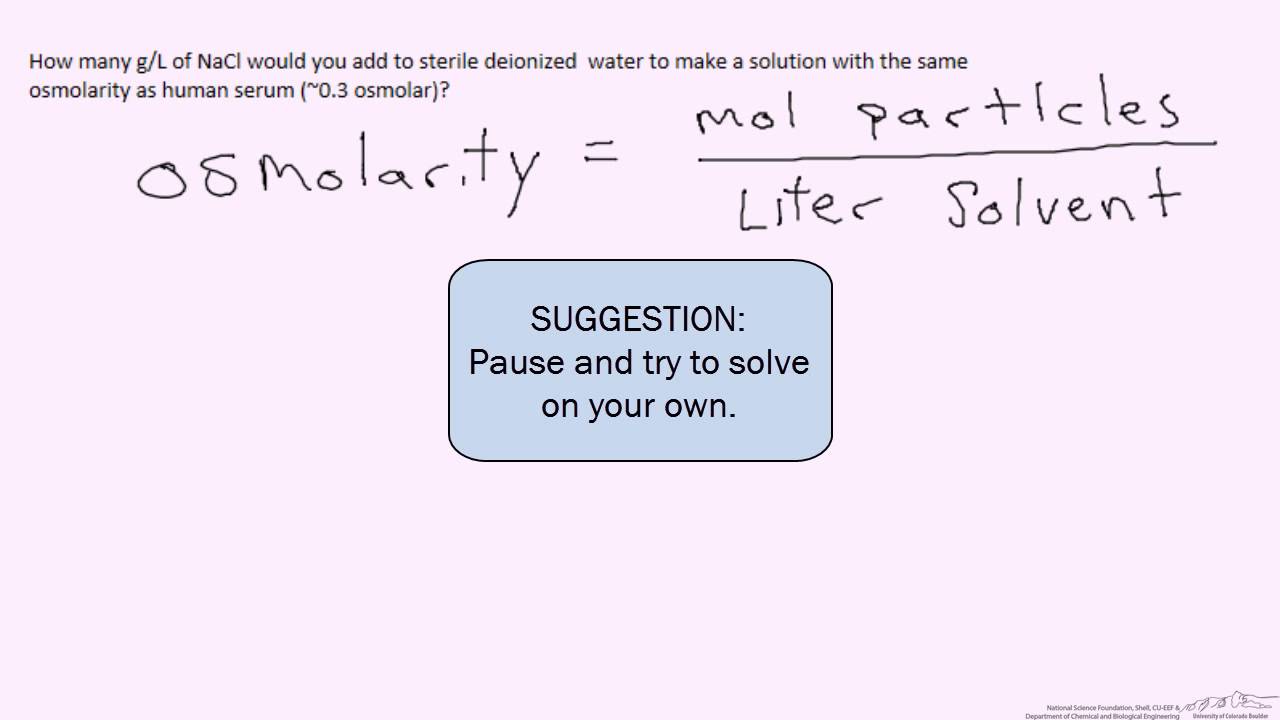
Osmolarity Example Bio Youtube
How To Calculate Osmolarity Easy To Calculate
3 Calculating Osmolarity Mathbench

Solved 1 A Write The Nernst Equation Below B Designate Chegg Com
:max_bytes(150000):strip_icc()/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)
Osmolarity And Osmolality In Chemistry
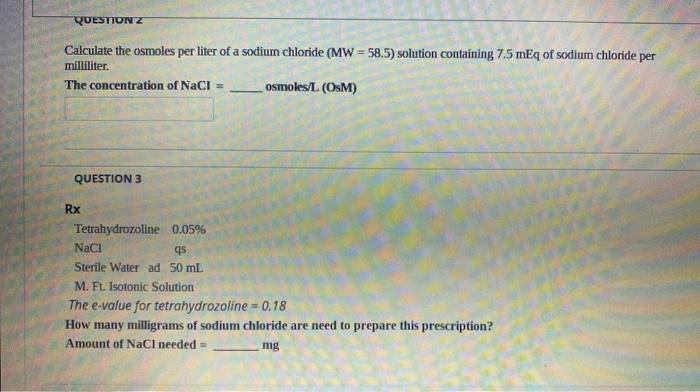
Solved Questiunz Calculate The Osmoles Per Liter Of A Sodium Chegg Com

Freezing Point Depression Youtube

Calculated Osmolality And Osmolality Gap Youtube

Osmolality Gap Calculation Avicenna School Youtube

Osmolarity Formula Calculation Units Video Lesson Transcript Study Com
How To Calculate Osmolarity Easy To Calculate

Hyperosmolar Hyperglycemic State Hhs Emcrit Project

Calculated Osmolality Youtube

Choice Of The Best Equation For Plasma Osmolality Calculation Comparison Of Fourteen Formulae Sciencedirect

Pdf Osmolality Revisited Deriving And Validating The Best Formula For Calculated Osmolality

Hyperosmolar Hyperglycemic State Hhs Emcrit Project